SpliSense’s cystic fibrosis treatment technology has been granted “orphan drug” designation by the US Food and Drug Administration and the European Medicines Agency, the Israeli biopharmaceutical company announced Tuesday.
Orphan drug designation is applied by the FDA and EMA to medicine and biologic technology intended to treat rare conditions.
“The orphan designations we received for our lead drug candidate both from the FDA and EMA are important milestones toward addressing the urgent, unmet needs of patients living with cystic fibrosis,” SpliSense CEO Gili Hart said in a press release. “We are looking forward to initiating the clinical study in the patient population in the US, Europe and Israel, which is aimed to be initiated later this year.”
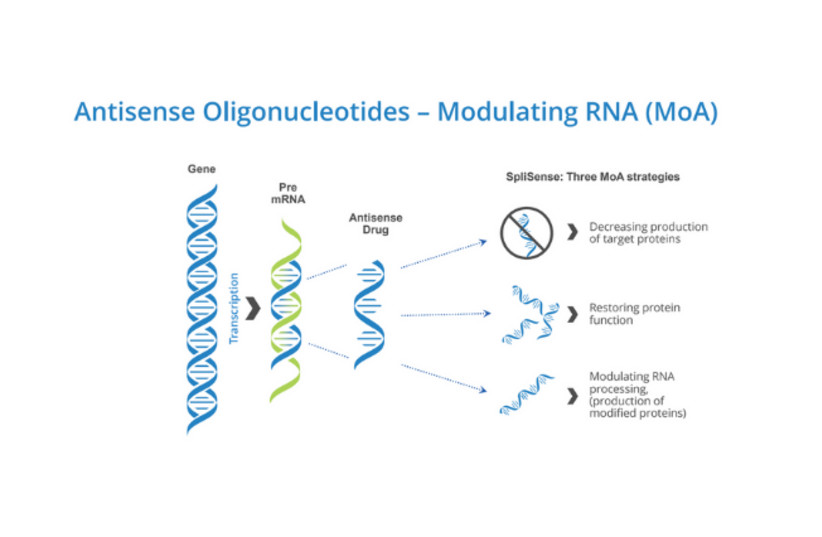
The product in question is an ASO (antisense oligonucleotide) called SPL84-23, a short and precisely targeted RNA strand that corrects a specific mutation that causes cystic fibrosis: the 3849 mutation in the CFTR gene. A dosage of these ASOs are inhaled into the lungs, where they are incorporated into the cells there and begin to produce corrected mRNA strands and normal proteins, potentially leading to significant improvement in lung function.
“In this case, we are masking the [mutated] sequences in the RNA,” said Prof. Batsheva Kerem, a genetics professor and researcher at the Hebrew University of Jerusalem, whose research is the foundation of SpliSense’s technology. “We skip over the sequences that were copied to the RNA in the wrong way.”
Kerem is a geneticist from the Hebrew University of Jerusalem who was part of the research team that first identified and cloned the CFTR gene.
Cystic fibrosis is a genetic disease that leads to respiratory infections and disabilities and affects more than 90,000 people worldwide. It is caused by mutations in the CFTR gene, leading to dysfunctional proteins.
While there are existing treatments for just over 80% of cystic fibrosis cases, there is a relatively small but significant percentage of patients who have no treatment options.
“We’re trying to treat those patients to give them hope and a clinical solution,” Hart said.
Many of the untreated patients are represented by the Cystic Fibrosis Foundation, a nonprofit organization in the US established to promote the development of a cure for cystic fibrosis and enable patients to live longer, healthier lives. In early 2021, the foundation invested $8.4 million in SpliSense’s treatment technology during a round that raised $28.5m.
“It was an assurance that they believe in our technology and our science,” Kerem said.
Hart said their drug would be administered to patients via inhalation on a weekly or every-other-week basis.
“By doing so, we believe it will generate a fully functional protein,” she said. “It will improve their lung function, reduce lung inflammation, enable them to breathe better and go back to normal life.”